
Full resolution (1,280 × 932 pixels, file size: 2.01 MB, MIME type: image/gif, looped, 6 frames, 18 s)
NASA projections of stratospheric ozone concentrations if chlorofluorocarbons had not been banned.Regulation
In 1978, the United States, Canada and Norway enacted bans on CFC-containing aerosol sprays
that are thought to damage the ozone layer. The European Community
rejected an analogous proposal to do the same. In the U.S.,
chlorofluorocarbons continued to be used in other applications, such as
refrigeration and industrial cleaning, until after the discovery of the
Antarctic ozone hole in 1985. After negotiation of an international treaty (the Montreal Protocol), CFC production was sharply limited beginning in 1987 and phased out completely by 1996.[citation needed]
Since that time, the treaty has been amended to ban CFC production
after 1995 in the developed countries, and later in developing. Today,
over 160 countries have signed the treaty. Beginning January 1, 1996,
only recycled and stockpiled CFCs will be available for use in developed
countries like the US. This production phaseout is possible because of
efforts to ensure that there will be substitute chemicals and
technologies for all CFC uses.[7]
On August 2, 2003, scientists announced that the depletion of the
ozone layer may be slowing down due to the international ban on CFCs.[8]
Three satellites and three ground stations confirmed that the upper
atmosphere ozone depletion rate has slowed down significantly during the
past decade. The study was organized by the American Geophysical Union.
Some breakdown can be expected to continue due to CFCs used by nations
which have not banned them, and due to gases which are already in the
stratosphere. CFCs have very long atmospheric lifetimes, ranging from 50
to over 100 years. It has been estimated that the ozone layer may not
recover until 2075.[9]
Compounds containing C–H bonds (such as hydrochlorofluorocarbons,
or HCFCs) have been designed to replace the function of CFCs. These
replacement compounds are more reactive and less likely to survive long
enough in the atmosphere to reach the stratosphere where they could
affect the ozone layer. While being less damaging than CFCs, HCFCs can
have a negative impact on the ozone layer, so they are also being phased
out.[10]
Wikipedia. Full resolution (SVG file, nominally 1,052 × 744 pixels, file size: 420 KB)
Full resolution (SVG file, nominally 1,052 × 744 pixels, file size: 420 KB)
The ozone layer is a layer in Earth's atmosphere containing relatively high concentrations of ozone (O3).
However, "relatively high," in the case of ozone, is still very small
with regard to ordinary oxygen, and is less than ten parts per million,
with the average ozone concentration in Earth's atmosphere being only
about 0.6 parts per million. The ozone layer is mainly found in the
lower portion of the stratosphere from approximately 20 to 30 kilometres (12 to 19 mi) above Earth, though the thickness varies seasonally and geographically.[1]
The ozone layer was discovered in 1913 by the French physicists Charles Fabry and Henri Buisson. Its properties were explored in detail by the British meteorologist G. M. B. Dobson, who developed a simple spectrophotometer (the Dobsonmeter)
that could be used to measure stratospheric ozone from the ground.
Between 1928 and 1958 Dobson established a worldwide network of ozone
monitoring stations, which continue to operate to this day. The "Dobson unit", a convenient measure of the columnar density of ozone overhead, is named in his honor.
The ozone layer absorbs 97–99% of the Sun's medium-frequency ultraviolet light (from about 200 nm to 315 nm wavelength), which potentially damages exposed life forms on Earth.[2]
8 February 2013
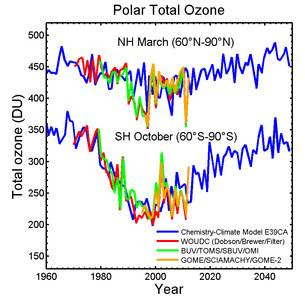
Satellites show that the recent ozone hole over Antarctica was the
smallest seen in the past decade. Long-term observations also reveal
that Earth’s ozone has been strengthening following international
agreements to protect this vital layer of the atmosphere.
According to the ozone sensor on Europe’s MetOp weather satellite, the hole over Antarctica in 2012 was the smallest in the last 10 years.
The instrument continues the long-term monitoring of atmospheric ozone started by its predecessors on the ERS-2 and Envisat satellites.
Since the beginning of the 1980s, an ozone hole has developed over Antarctica during the southern spring – September to November – resulting in a decrease in ozone concentration of up to 70%.
According to the ozone sensor on Europe’s MetOp weather satellite, the hole over Antarctica in 2012 was the smallest in the last 10 years.
The instrument continues the long-term monitoring of atmospheric ozone started by its predecessors on the ERS-2 and Envisat satellites.
Since the beginning of the 1980s, an ozone hole has developed over Antarctica during the southern spring – September to November – resulting in a decrease in ozone concentration of up to 70%.
Ozone depletion is more extreme in Antarctica than at the North Pole
because high wind speeds cause a fast-rotating vortex of cold air,
leading to extremely low temperatures. Under these conditions,
human-made chlorofluorocarbons – CFCs – have a stronger effect on the
ozone, depleting it and creating the infamous hole.
Over the Arctic, the effect is far less pronounced because the northern hemisphere’s irregular landmasses and mountains normally prevent the build-up of strong circumpolar winds.
Reduced ozone over the southern hemisphere means that people living there are more exposed to cancer-causing ultraviolet radiation.
International agreements on protecting the ozone layer – particularly the Montreal Protocol – have stopped the increase of CFC concentrations, and a drastic fall has been observed since the mid-1990s.
However, the long lifetimes of CFCs in the atmosphere mean it may take until the middle of this century for the stratosphere’s chlorine content to go back to values like those of the 1960s.
The evolution of the ozone layer is affected by the interplay between atmospheric chemistry and dynamics like wind and temperature.
If weather and atmospheric conditions show unusual behaviour, it can result in extreme ozone conditions – such as the record low observed in spring 2011 in the Arctic – or last year’s unusually small Antarctic ozone hole.
Over the Arctic, the effect is far less pronounced because the northern hemisphere’s irregular landmasses and mountains normally prevent the build-up of strong circumpolar winds.
Reduced ozone over the southern hemisphere means that people living there are more exposed to cancer-causing ultraviolet radiation.
International agreements on protecting the ozone layer – particularly the Montreal Protocol – have stopped the increase of CFC concentrations, and a drastic fall has been observed since the mid-1990s.
However, the long lifetimes of CFCs in the atmosphere mean it may take until the middle of this century for the stratosphere’s chlorine content to go back to values like those of the 1960s.
The evolution of the ozone layer is affected by the interplay between atmospheric chemistry and dynamics like wind and temperature.
If weather and atmospheric conditions show unusual behaviour, it can result in extreme ozone conditions – such as the record low observed in spring 2011 in the Arctic – or last year’s unusually small Antarctic ozone hole.
To understand these complex processes better, scientists rely on a long
time series of data derived from observations and on results from
numerical simulations based on complex atmospheric models.
Although ozone has been observed over several decades with multiple
instruments, combining the existing observations from many different
sensors to produce consistent and homogeneous data suitable for
scientific analysis is a difficult task.
Within the ESA Climate Change Initiative, harmonised ozone climate data
records are generated to document the variability of ozone changes
better at different scales in space and time.
With this information, scientists can better estimate the timing of the
ozone layer recovery, and in particular the closure of the ozone hole.
Chemistry climate models show that the ozone layer may be building up,
and the hole over Antarctica will close in the next decades.
Guillermo Gonzalo Sánchez Achutegui
ayabaca@gmail.com
ayabaca@hotmail.com
ayabaca@yahoo.com

No hay comentarios:
Publicar un comentario