 |
|
| Research site in Netarts Bay, Oregon, at low tide; rows of bags contain seed oysters. Credit: R. Mabardy, OSU |
| Download the high-resolution JPG version of the image. (4 MB) |
 |
| Oysters at hatcheries in Oregon are showing the effects of ocean acidification. Credit: OSU |
Download the high-resolution JPG version of the image. (83 KB) |
 |
| Oyster larvae are placed into growing tanks at Oregon's Whiskey Creek Shellfish Hatchery. Credit: Lynn Ketchum, OSU |
| Download the high-resolution JPG version of the image. (1.9 MB) |
 |
| Researcher behind screen with oyster larvae at the Whiskey Creek Shellfish Hatchery. Credit: Lynn Ketchum, OSU |
| Download the high-resolution JPG version of the image. (16 KB) |
 |
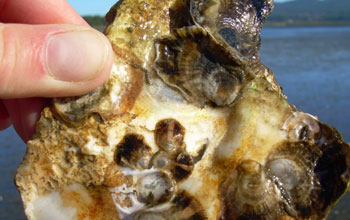
| Pacific oysters, healthy at Taylor Shellfish Farms; will the oysters survive ocean acidification? Credit: Taylor Shellfish Farm, NOAA |
| Download the high-resolution JPG version of the image. (134 KB) |
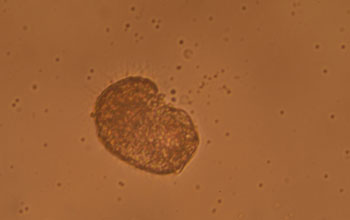 |
| Pacific oyster embryo, seen under a light microscope. Formation of first shell has just begun. Credit: G. Waldbusser, OSU |
| Download the high-resolution JPG version of the image. (2 MB) | ||||||||||||||||||||||||||
In World Oceans Month, there's mixed news for the Pacific Northwest oyster industry.
For
the past several years, it has struggled with significant losses due to
ocean acidification. Oyster larvae have had mortality rates high
enough to render production no longer economically feasible.
Now a new study documents why oysters appear so sensitive to increasing acidity, but also offers some hope for the future.
It
isn't necessarily a case of acidic water dissolving the oysters'
shells, scientists say. It's water high in carbon dioxide altering
shell formation rates, energy usage and, ultimately, the growth and
survival of young .
"The failure of oyster seed production
in Northwest Pacific coastal waters is one of the most graphic examples
of ocean acidification effects on important commercial shellfish," said
Dave Garrison, program director in the National Science Foundation's
(NSF) Division of Ocean Sciences.
NSF funded the study through its
Ocean Acidification Program, part of NSF's Science, Engineering and
Education for Sustainability programs.
"This research is among the
first to identify the links among organism physiology, ocean carbonate
chemistry and oyster seed mortality," said Garrison.
Results of the study are online in the journal Geophysical Research Letters, published by the American Geophysical Union.
"From
the time eggs are fertilized, Pacific oyster larvae precipitate roughly
90 percent of their body weight as a calcium carbonate shell within 48
hours," said George Waldbusser, an Oregon State University marine
ecologist and lead author of the paper.
"Young oysters rely solely on the energy they derive from the egg because they have not yet developed feeding organs."
During
exposure to increasing carbon dioxide in acidified water, however, it
becomes more energetically expensive for organisms like oysters to build
shells.
Adult oysters and other bivalves may grow more slowly
when exposed to rising carbon dioxide levels. But larvae in the first
two days of life do not have the luxury of delayed growth.
"They
must build their first shell quickly on a limited amount of energy--and
along with the shell comes the organ to capture external food," said
Waldbusser.
"It becomes a death race of sorts. Can the oyster
build its shell quickly enough to allow its feeding mechanism to develop
before it runs out of energy from the egg?"
The results are
important, marine scientists say, because they document for the first
time the links among shell formation rate, available energy, and
sensitivity to acidification.
The researchers say that the faster
the rate of shell formation, the more energy is needed. Oyster embryos
building their first shells need "to make a lot of shell on short
order," said Waldbusser.
"As the carbon dioxide in seawater
increases, but before waters become corrosive, calcium carbonate
precipitation requires more energy to maintain higher rates of shell
formation during this early stage."
The researchers worked with
Whiskey Creek Shellfish Hatchery in Netarts Bay, Ore. They found that
on the second day of life, 100 percent of the larval tissue growth was
from egg-derived carbon.
"The oyster larvae were still relying on
egg-derived energy until they were 11 days old," said Elizabeth Brunner
of Oregon State University and a co-author of the paper.
The earliest shell material in the larvae contained the greatest amount of carbon from the surrounding waters.
Increasing
amounts of carbon from respiration were incorporated into shells after
the first 48 hours, indicating an ability to isolate and control the
shell surfaces where calcium carbonate is being deposited.
Waldbusser
notes that adult bivalves are well-adapted to growing shell in
conditions that are more acidified, and have evolved several mechanisms
to do so.
These include use of organic molecules to organize and
facilitate the formation of calcium carbonate, pumps that remove acid
from the calcifying fluids, and outer shell coatings that protect
minerals to some degree from surrounding waters.
Waldbusser said
that the results help explain previous findings at the Whiskey Creek
Hatchery of larval sensitivity to waters that are high in carbon dioxide
but not corrosive to calcium carbonate.
They also explain
carryover effects later in larval life of exposure to high carbon
dioxide, similar to human neonatal nutrition effects.
The
discovery may be good news, scientists say, because there are
interventions that can be done at hatcheries that may offset some of the
effects of ocean acidification.
Some hatcheries have begun
"buffering" water for larvae--essentially adding antacids to incoming
waters--including the Whiskey Creek Hatchery and the Taylor Shellfish
Farms in Washington.
The study provides a scientific foundation for the target level of buffering.
"You
can make sure that eggs have more energy before they enter the larval
stage," said Waldbusser, "so a well-balanced adult diet may help larval
oysters cope better with the stress of acidified water."
-NSF-
Media Contacts
Cheryl Dybas, NSF (703) 292-7734
cdybas@nsf.gov
Mark Floyd, Oregon State University (541) 737-0788 mark.floyd@oregonstate.edu Peter Weiss, American Geophysical Union (202) 777-7507 pweiss@agu.org
Related WebsitesNSF News Release: Ocean Acidification Linked With Larval Oyster Failure in Hatcheries:
http://www.nsf.gov/news/news_summ.jsp?cntn_id=123822
NSF Science, Engineering and Education for Sustainability Programs: http://www.nsf.gov/sees NSF Publication: Discoveries in Sustainability:
The National Science Foundation (NSF) is an independent federal
agency that supports fundamental research and education across all
fields of science and engineering. In fiscal year (FY) 2012, its budget
was $7.0 billion. NSF funds reach all 50 states through grants to nearly
2,000 colleges, universities and other institutions. Each year, NSF
receives about 50,000 competitive requests for funding, and makes about
11,500 new funding awards. NSF also awards about $593 million in
professional and service contracts yearly.
Useful NSF Web Sites:
NSF Home Page:
http://www.nsf.gov
NSF News:
http://www.nsf.gov/news/
For the News Media:
http://www.nsf.gov/news/newsroom.jsp
Science and Engineering Statistics:
http://www.nsf.gov/statistics/
Awards Searches:
Guillermo Gonzalo Sánchez Achutegui
ayabaca@gmail.com
ayabaca@hotmail.com
ayabaca@yahoo.com
|
No hay comentarios:
Publicar un comentario